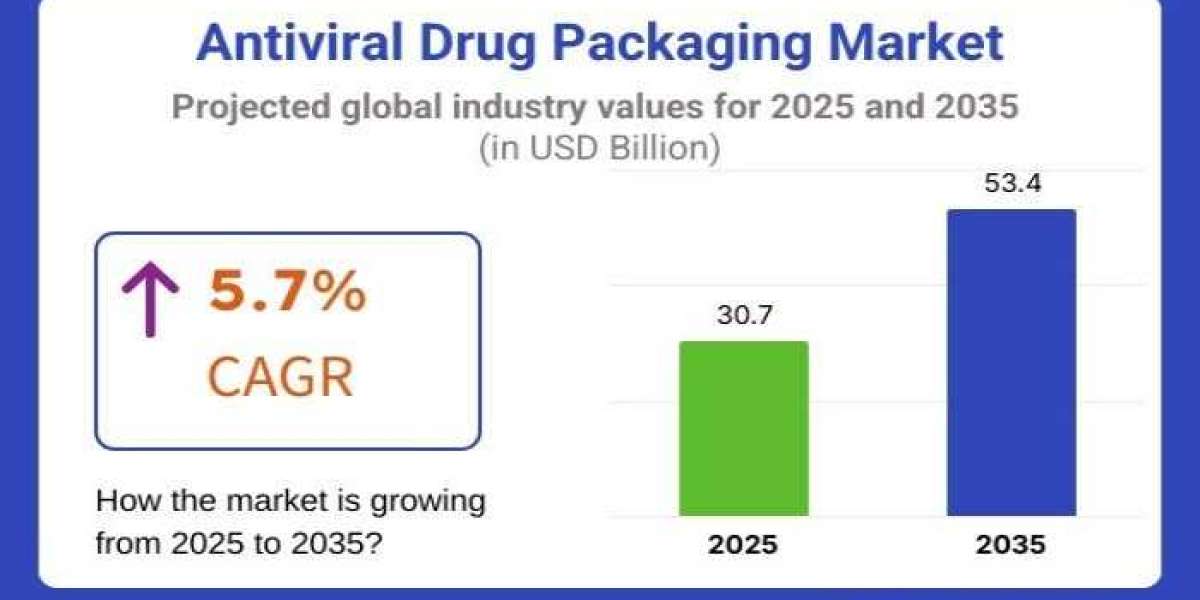
The global Antiviral Drug Packaging Market is entering a decade of sustained innovation, expanding from USD 30.7 billion in 2025 to an estimated USD 53.4 billion by 2035, at a CAGR of 5.7%. The industry’s rapid expansion is closely tied to the rising global burden of viral diseases—including hepatitis, HIV, influenza, and emerging outbreak-related infections—which continues to elevate demand for secure, sterile, regulation-compliant pharmaceutical packaging.
The rising consumption of antiviral medicines is pushing manufacturers toward high-barrier, contamination-free, tamper-proof, and sustainable packaging materials. The focus is shifting toward pharmaceutical-grade packaging that protects drug integrity, enhances patient usability, and complies with increasingly stringent global regulatory frameworks.
Subscribe for Year-Round Insights → Stay ahead with quarterly and annual data updates – https://www.futuremarketinsights.com/reports/sample/rep-gb-13397
Market Drivers: Compliance, Hygiene, and Sustainability
With regulators such as the FDA, EMA, and national health bodies tightening packaging standards, antiviral drug manufacturers are accelerating the adoption of child-resistant, tamper-evident, and sterilizable materials. Advanced solutions such as pre-filled syringes, high-barrier blister films, smart labels, and moisture-resistant vials are gaining traction across global supply chains.
Sustainability is emerging as a major industry priority. Manufacturers are investing in biodegradable blister packs, recyclable glass vials, plant-based polymers, and eco-conscious secondary packaging to meet corporate ESG goals and consumer expectations.
Simultaneously, the expansion of healthcare infrastructure in developing economies is offering new opportunities for technology-driven packaging solutions.
Regional Market Highlights
North America
North America remains one of the largest and most regulated markets. Strong pharmaceutical RD, rapid drug commercialization, and FDA-led compliance standards continue to fuel demand for innovative, patient-safe, and sustainable antiviral packaging. Adoption of smart packaging, pre-filled systems, and serialization technologies is expanding rapidly, driven primarily by the U.S.
Europe
Europe’s antiviral packaging demand is reinforced by strict EU pharmaceutical safety mandates and a high consumer preference for sustainable solutions. With 19% of consumers actively avoiding wasteful packaging, manufacturers in Germany, France, and the UK are prioritizing recyclable, recyclable, and child-resistant designs. Growth in biosimilars and cold-chain logistics is further stimulating innovation.
Asia-Pacific
The Asia-Pacific region is the fastest-growing market, underpinned by strong pharmaceutical manufacturing in China, India, and Japan. Increasing infectious disease prevalence, expanding CMOs, and supportive government healthcare initiatives are strengthening the region’s relevance. Cost-efficient, eco-friendly materials are gaining momentum across APAC pharmaceutical hubs.
Challenges: Regulatory Pressure and Material Complexity
The industry faces challenges related to stringent regulatory compliance, sterility maintenance, and the need for high-performance materials that defend drugs from light, oxygen, and moisture. Liquid formulations and biologics demand advanced, contamination-free packaging, increasing production costs and material research requirements.
Opportunities: Smart, Eco-Friendly, and Tamper-Proof Solutions
The next decade will see explosive adoption of:
- Biodegradable and recyclable packaging
- Tamper-evident films and seals
- Smart labels, NFC tags, and RFID-enabled traceability
- Personalized packaging for complex antiviral formulations
These advancements ensure product authenticity, enhance patient safety, and support global anti-counterfeiting efforts.
Market Evolution: 2020–2024 vs. 2025–2035
From 2020 to 2024, the market observed significant adoption of single-use sterile packaging, child-proof closures, and high-barrier plastics. Between 2025 and 2035, sustainability, energy efficiency, and smart tracking technologies are expected to dominate, aligned with global standards for drug integrity and environmental responsibility.
Country-Specific CAGR (2025–2035)
- USA: 5.9%
- United Kingdom: 5.6%
- European Union: 5.8%
- Japan: 5.7%
- South Korea: 5.8%
Segment Outlook
Primary Packaging Leads
Demand is highest for blister packs, vials, prefilled syringes, ampoules, and unit-dose systems, which provide direct drug contact and ensure sterility. Over 65% of antiviral drugs rely on high-barrier primary containment materials.
Secondary Packaging Gains Importance
Secondary packaging such as folding cartons, corrugated boxes, and serialized labels plays a central role in logistics, branding, and anti-counterfeiting compliance.
Competitive Landscape
The market is moderately consolidated, with leading players focusing on high-barrier, sustainable, and patient-centric packaging solutions.
- Amcor Plc (18-22%)
- West Pharmaceutical Services, Inc.(12-16%)
- Gerresheimer AG (10-14%)
- Schott AG (8-12%)
- Berry Global Inc. (5-9%)
Why FMI: https://www.futuremarketinsights.com/why-fmi
About Future Market Insights (FMI)
Future Market Insights, Inc. (FMI) is an ESOMAR-certified, ISO 9001:2015 market research and consulting organization, trusted by Fortune 500 clients and global enterprises. With operations in the U.S., UK, India, and Dubai, FMI provides data-backed insights and strategic intelligence across 30+ industries and 1200 markets worldwide.